How much do you know about the import and export of medical
How much do you know about the import and export of medical devices?
1、 What is a medical device?
Medical devices refer to instruments, equipment, instruments, in vitro diagnostic reagents and calibrators, materials, and other similar or related items directly or indirectly used in the human body, including required computer software; Its effectiveness is mainly obtained through physical and other means, not through pharmacology, immunology, or metabolism, or although these methods are involved, they only have auxiliary effects; Its purpose is to:
(1) Diagnosis, prevention, monitoring, treatment or relief of diseases;
(2) Diagnosis, monitoring, treatment, relief or functional compensation of injuries;
(3) Testing, substitution, regulation, or support of physiological structures or processes;
(4) Support or maintenance of life;
(5) Pregnancy control;
(6) Provide information for medical or diagnostic purposes by examining samples from the human body.
2、 Classification of medical devices
1. The country implements classified management of medical devices according to their degree of risk
The first category refers to medical devices that are sufficient to ensure their safety and effectiveness through routine management:
Such as surgical instruments (knives, scissors, forceps, forceps, hooks), scraping plates, medical X-ray films, surgical gowns, surgical caps, examination gloves, gauze bandages, drainage bags, etc.
The second category refers to medical devices whose safety and effectiveness should be controlled:
Such as medical suture needle, blood pressure needle, thermometer, electrocardiograph, electroencephalograph, microscope, acupuncture and moxibustion needle, biochemical analysis system, hearing aid, ultrasonic disinfection equipment, non absorbable suture, condom, etc.
The third category of medical devices that have potential hazards to the human body and must be strictly controlled for their safety and effectiveness:
Such as implantable pacemakers, corneal contact lenses, artificial lenses, ultra economical tumor focusing knives, hemodialysis devices, implantation equipment, vascular stents, comprehensive anesthesia machines, dental implant materials, medical absorbable sutures, intravascular catheters, etc.
Kind reminder: When evaluating the risk level of medical devices, factors such as the expected purpose, structural characteristics, and usage methods of the medical device should be considered.
2. Classification Catalogue of Medical Products
The former State Food and Drug Administration organized the revision of the "Classification Catalogue of Medical Devices", which was officially released in September 2017 and implemented on August 1, 2018. The revised "Classification Catalogue of Medical Devices" consists of 22 subcategories, refining and adjusting 260 product categories into 206 primary product categories and 1157 secondary product categories, forming a three-level directory hierarchy.
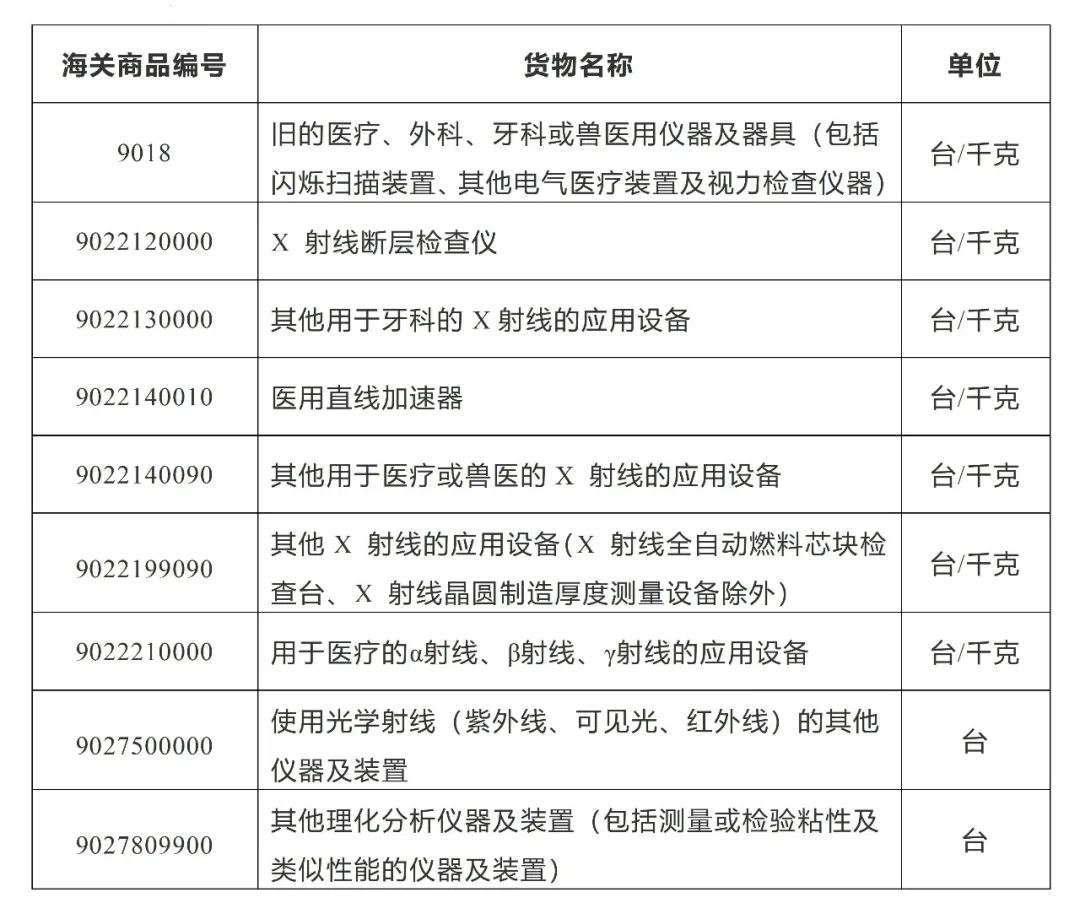
What are the customs tariff codes for medical device products?
The customs tariff codes for medical devices are mainly concentrated in tariff codes 9018, 9021, 9022, 9402, and other items. During import and export customs declaration and export tax refund, search for corresponding customs tariff codes based on different products. The following is a list of customs tariff codes and names for some medical device products for reference.
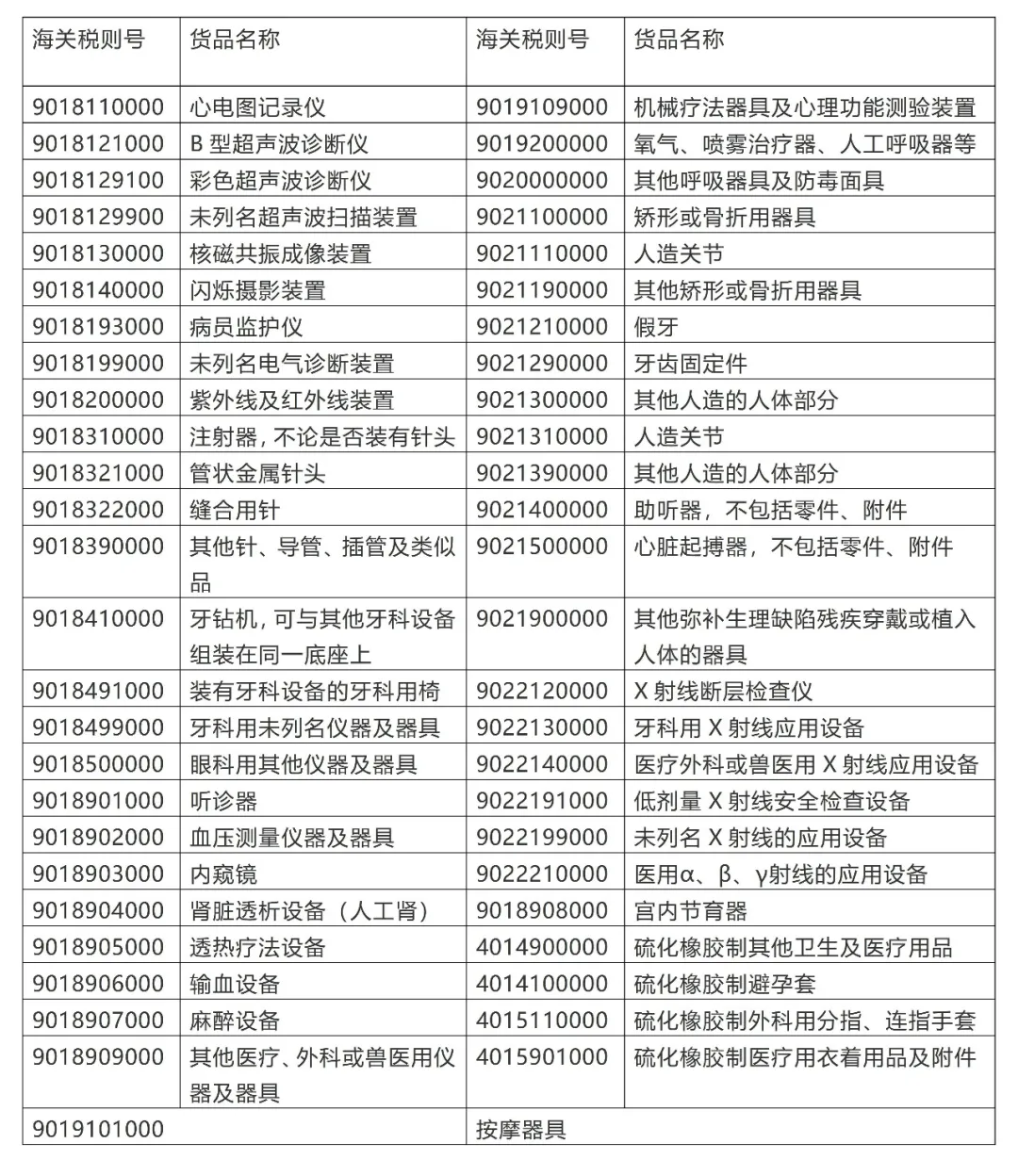
3、 Medical Device Filing and Registration
The first category of medical devices is subject to product registration management, while the second and third categories of medical devices are subject to product registration management.
For overseas filing/registration applicants who export medical devices to China, their designated domestic enterprise legal representative shall submit the filing/registration application materials and proof of approval from the competent authorities of the country (region) where the filing/registration applicant is located to the drug regulatory department of the State Council for the listing and sale of the medical device. Innovative medical devices that have not been listed overseas may not be required to submit proof documents from the competent authorities of the country (region) where the registrant is located that allow the medical device to be listed for sale.
4、 Import and export of medical device products
1. Export of medical devices
Enterprises exporting medical devices should ensure that their exported medical devices meet the requirements of the importing country (region), and generally do not implement commodity inspection.
It should be noted that according to Announcement No. 53 of 2020 and Announcement No. 124 of 2020 of the General Administration of Customs, export commodity inspection will be carried out on medical supplies under five 10 digit commodity numbers, including 6307900010.
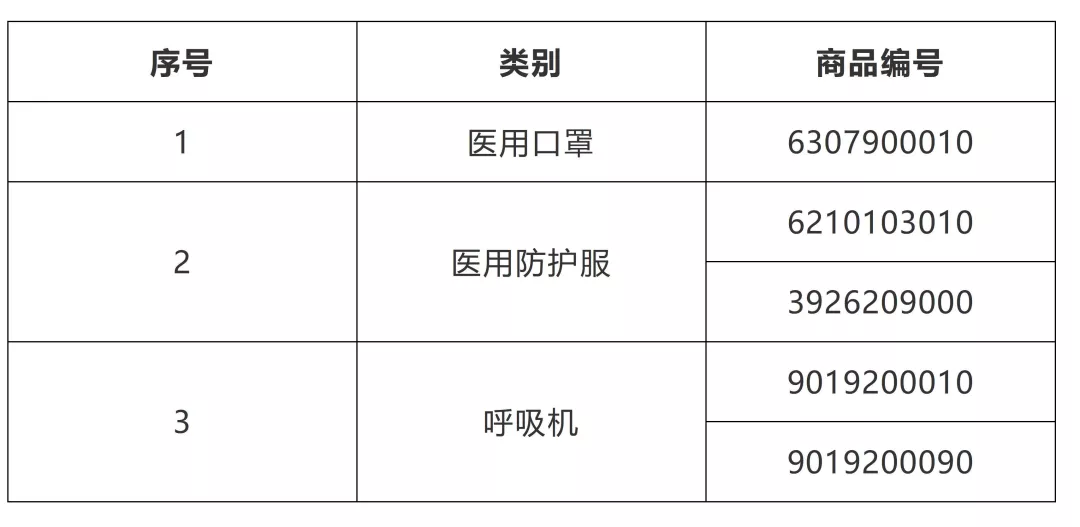
Kind reminder: For dangerous goods under commodity numbers 3005901000 and 3005909000, and dangerous chemicals under commodity numbers 3808940010, the inspection and supervision requirements for exported dangerous goods or exported dangerous chemicals shall be followed.
2. Imported medical devices
The imported medical devices should be registered or registered medical devices, and the validity period of the medical device registration certificate is 5 years. If the registration needs to be renewed after the expiration of the validity period, an application for renewal of registration should be submitted to the original registration department 6 months before the expiration of the validity period.
Regarding the validity period of the medical device registration certificate. Notice of the State Administration of Food and Drug Administration on the Implementation of the Management Measures for the Registration of Medical Devices and In Vitro Diagnostic Reagents (Food and Drug Administration, 2015 No. 247): Medical devices approved for registration refer to medical devices that are consistent with the limited content of the medical device registration certificate and attachments and are produced within the validity period of the medical device registration certificate.
Medical institutions that urgently need to import a small amount of Class II and Class III medical devices due to clinical needs may import them with the approval of the drug regulatory department of the State Council or the people's governments of provinces, autonomous regions, and municipalities authorized by the State Council. Imported medical devices should be used for specific medical purposes within designated medical institutions.
Customs conducts online verification of the electronic data of imported medical device registration certificates (including medical device registration certificates and Class I medical device registration vouchers) and the electronic data of imported medical device product declaration forms.
When declaring imported medical devices, contracts, invoices, packing lists, and medical device registration certificates should be provided. Imported medical devices should have Chinese instructions and labels. The instructions and labels shall comply with the provisions of this Regulation and relevant mandatory standards, and shall specify the origin of the medical device and the name, address, and contact information of the domestic enterprise legal person designated by the overseas medical device registrant and registrant in China.
Customs shall conduct inspections on imported medical devices in accordance with the law; Those that fail the inspection shall not be imported.
(1) Verify whether the product information, declaration information, and admission information inspected on site are consistent.
(2) Verify whether the relevant information on the nameplate of imported medical devices is consistent with the registered or registered manufacturer, product name, and model specifications.
5、 Special requirements
1. Special requirements for imported cardiac pacemakers
The General Administration of Customs designates customs to conduct inspection and supervision on imported cardiac pacemakers, and is responsible for designating nationally recognized medical device testing institutions to conduct testing on imported cardiac pacemakers.
Beijing Customs, Shanghai Customs, and Haikou Customs are designated inspection and implementation agencies for imported pacemakers.
2. Special requirements for imported ventilators
The customs carry out key supervision on imported high-risk ventilators, and relevant inspection and supervision work is carried out by customs in Beijing, Tianjin, Dalian, Shanghai, Qingdao, Wuhan, Guangzhou, and other places.
3. Import donated medical devices
Foreign donors are prohibited from carrying items listed in the "Catalogue of Prohibited Import Goods" in medical devices donated to China. The donated medical devices should be new and have been registered in China. Prohibited items such as harmful environment, public health, social morality, and political infiltration are not allowed to be carried.
Note: List of prohibited imports of medical device products
Prohibit the import of expired, expired, and obsolete medical devices that have already been used.
According to Announcement No. 106 of the General Administration of Customs of the Ministry of Commerce in 2018, the catalog of used mechanical and electrical products prohibited from import has been adjusted and will be implemented from January 1, 2019.
本文来源:http://www.jtia56.cn/list_67/450.html
本文标题:How much do you know about the import and export of medical
注:本文部分图文来源于网络,如有侵权联系我们删除,谢谢!
【 Case Analysis 】 Tianjin Port Import Vehicle Milling Com 【 Customs Knowledge 】 Tips for Imported Pressure Vessels
迦泰通(海关AEO高级认证企业)-服务范围
迦泰通19年进出口通关经验,10+分公司,支持全国进口申报;是海关AEO高级认证企业,专注全球门到门,一站式进口代理清关服务!我司业务范围:国际运输、进口报关清关、仓储配送、代签外贸合同与付汇、暂时进出口等。全国免费咨询电话tel:18521306667
【相关推荐】
- 了解详情 > 【 Customs Knowledge 】 Teach you how to import and export
- 了解详情 > How much do you know about the import and export of medical
- 了解详情 > Customs Knowledge: Import of Medical Devices
- 了解详情 > 【 Case Analysis 】 Taiwan Automation Processing Center Equ
- 了解详情 > 【 Case Analysis 】 Tianjin Port Import Vehicle Milling Com
- 了解详情 > 【 Customs Knowledge 】 Key Points for Customs Clearance of






